 Rivista in lingua italiana
Rivista in lingua italiana
riservata ai Soci SISA
Ultimo numero:
Anno 16 • N.1/2025
SISANews
Efficacia della polipillola nella prevenzione primaria e secondaria di malattie cardiovascolari
Il trial PolyIran, uno studio a due bracci, pragmatico, randomizzato a cluster, innestato all'interno del Golestan Cohort Study (GCS), uno studio di coorte di 50.045 partecipanti di età compresa tra 40 e 75 anni della provincia del Golestan in Iran, ha valutato efficacia e sicurezza di una polipillola di 4 farmaci (aspirina, atorvastatina, idroclorotiazide ed enalapril o valsartan) nella prevenzione primaria e secondaria delle malattie cardiovascolari. I soggetti sono stati assegnati (1:1) a un insieme di interventi preventivi non farmacologici (educazione a uno stile di vita sano, ad es. dieta sana con basso contenuto di sale, zucchero e grassi, esercizio fisico, controllo del peso e astinenza dal fumo) o a un gruppo che includeva anche l’assunzione della polipillola una volta al giorno. I partecipanti sono stati seguiti per 60 mesi. L’outcome primario era un composito di eventi cardiovascolari maggiori. Sono stati arruolati 6838 soggetti nello studio - 3417 nel gruppo di cure minime e 3421 nel gruppo polipillola. Durante il follow-up, 301 (8,8%) partecipanti nel gruppo di cure minime hanno avuto eventi cardiovascolari maggiori, rispetto al 202 (5,9%) partecipanti nel gruppo polipillola (HR 0,66; IC 95% 0,55-0,80). Non è stata trovata alcuna interazione statisticamente significativa con la presenza (HR 0,61; 0,49-0,75) o l'assenza di malattie cardiovascolari preesistenti (HR 0,80; 0,51-1,12; p per l’interazione 0,19). Se valutata nei soli partecipanti con elevata aderenza, la riduzione dell’outcome primario era persino maggiore rispetto al gruppo di cure minime (HR 0,43; 0,33-0,55). La frequenza degli eventi avversi era simile tra i due gruppi di studio. In conclusione, l'uso della polipillola è risultato efficace nella prevenzione di eventi cardiovascolari maggiori, e potrebbe essere considerato come un’ulteriore opzione per il controllo delle malattie cardiovascolari.
![]()
Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial
Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, Ostovaneh MR, Nateghi A, Majed M, Navabakhsh B, Merat S, Pourshams A, Nalini M, Malekzadeh F, Sadeghi M, Mohammadifard N, Sarrafzadegan N, Naemi-Tabiei M, Fazel A, Brennan P, Etemadi A, Boffetta P, Thomas N, Marshall T, Cheng KK, Malekzadeh R.
Lancet 2019; 394:672-683
BACKGROUND: A fixed-dose combination therapy (polypill strategy) has been proposed as an approach to reduce the burden of cardiovascular disease, especially in low-income and middle-income countries (LMICs). The PolyIran study aimed to assess the effectiveness and safety of a four-component polypill including aspirin, atorvastatin, hydrochlorothiazide, and either enalapril or valsartan for primary and secondary prevention of cardiovascular disease.
METHODS: The PolyIran study was a two-group, pragmatic, cluster-randomised trial nested within the Golestan Cohort Study (GCS), a cohort study with 50 045 participants aged 40-75 years from the Golestan province in Iran. Clusters (villages) were randomly allocated (1:1) to either a package of non-pharmacological preventive interventions alone (minimal care group) or together with a once-daily polypill tablet (polypill group). Randomisation was stratified by three districts (Gonbad, Aq-Qala, and Kalaleh), with the village as the unit of randomisation. We used a balanced randomisation algorithm, considering block sizes of 20 and balancing for cluster size or natural log of the cluster size (depending on the skewness within strata). Randomisation was done at a fixed point in time (Jan 18, 2011) by statisticians at the University of Birmingham (Birmingham, UK), independent of the local study team. The non-pharmacological preventive interventions (including educational training about healthy lifestyle-eg, healthy diet with low salt, sugar, and fat content, exercise, weight control, and abstinence from smoking and opium) were delivered by the PolyIran field visit team at months 3 and 6, and then every 6 months thereafter. Two formulations of polypill tablet were used in this study. Participants were first prescribed polypill one (hydrochlorothiazide 12·5 mg, aspirin 81 mg, atorvastatin 20 mg, and enalapril 5 mg). Participants who developed cough during follow-up were switched by a trained study physician to polypill two, which included valsartan 40 mg instead of enalapril 5 mg. Participants were followed up for 60 months. The primary outcome-occurrence of major cardiovascular events (including hospitalisation for acute coronary syndrome, fatal myocardial infarction, sudden death, heart failure, coronary artery revascularisation procedures, and non-fatal and fatal stroke)-was centrally assessed by the GCS follow-up team, who were masked to allocation status. We did intention-to-treat analyses by including all participants who met eligibility criteria in the two study groups. The trial was registered with ClinicalTrials.gov, number NCT01271985.
FINDINGS: Between Feb 22, 2011, and April 15, 2013, we enrolled 6838 individuals into the study-3417 (in 116 clusters) in the minimal care group and 3421 (in 120 clusters) in the polypill group. 1761 (51·5%) of 3421 participants in the polypill group were women, as were 1679 (49·1%) of 3417 participants in the minimal care group. Median adherence to polypill tablets was 80·5% (IQR 48·5-92·2). During follow-up, 301 (8·8%) of 3417 participants in the minimal care group had major cardiovascular events compared with 202 (5·9%) of 3421 participants in the polypill group (adjusted hazard ratio [HR] 0·66, 95% CI 0·55-0·80). We found no statistically significant interaction with the presence (HR 0·61, 95% CI 0·49-0·75) or absence of pre-existing cardiovascular disease (0·80; 0·51-1·12; pinteraction=0·19). When restricted to participants in the polypill group with high adherence, the reduction in the risk of major cardiovascular events was even greater compared with the minimal care group (adjusted HR 0·43, 95% CI 0·33-0·55). The frequency of adverse events was similar between the two study groups. 21 intracranial haemorrhages were reported during the 5 years of follow-up-ten participants in the polypill group and 11 participants in the minimal care group. There were 13 physician-confirmed diagnoses of upper gastrointestinal bleeding in the polypill group and nine in the minimal care group.
INTERPRETATION: Use of polypill was effective in preventing major cardiovascular events. Medication adherence was high and adverse event numbers were low. The polypill strategy could be considered as an additional effective component in controlling cardiovascular diseases, especially in LMICs.
FUNDING: Tehran University of Medical Sciences, Barakat Foundation, and Alborz Darou.
Area Soci
Eventi
39° Congresso Nazionale
 39° Congresso Nazionale
39° Congresso NazionaleRoma, 23-25 novembre 2025
Save the date




 Spring Meeting Gruppi Giovani SID, SIGG, SIIA, SIMI, SIPREC, SISA
Spring Meeting Gruppi Giovani SID, SIGG, SIIA, SIMI, SIPREC, SISARimini, 6-8 aprile 2025
[continua a leggere]
 SISA LIPID ACADEMY - Corso avanzato di lipidologia clinica
SISA LIPID ACADEMY - Corso avanzato di lipidologia clinicaModena, 4-5 Luglio 2024
[continua a leggere]Giornale Italiano Arteriosclerosi
HoFH today
 Rivista Italiana della
Rivista Italiana della
Ipercolesterolemia
Familiare Omozigote
Anno 6 • N.1/2024
Rivista NMCD
Diateca
[continua a leggere]
[continua a leggere]
Newsletter
il vostro indirizzo di posta elettronica
Progetto LIPIGEN
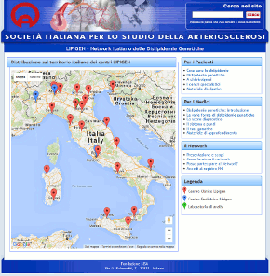
Nuovo sito dedicato al Progetto LIPIGEN
Progetto LIPIGEN - Vecchio portale
E' necessario essere loggati come utente
Lipigen per poter accedere alla pagina
PROject Statin Intolerance SISA
PROSISA – PROject Statin Intolerance SISA
E' necessario essere loggati come utente
PROSISA per poter accedere alla pagina
GILA - Lipoprotein Aferesi
Gruppo Interdisciplinare Lipoprotein Aferesi
(Accesso Gruppo GILA-Lipoprotein Aferesi)
E' necessario essere loggati come utente del Gruppo GILA per poter accedere
Gruppo Interdisciplinare Lipoprotein Aferesi
(Documentazione ad accesso libero)
Pagina informativa per medici e pazienti










