 Rivista in lingua italiana
Rivista in lingua italiana
riservata ai Soci SISA
Ultimo numero:
Anno 16 • N.1/2025
Abstract
Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy
Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R.Lancet 2012;380:29-36
BACKGROUND: Inhibition of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) resulted in large reductions of low-density lipoprotein cholesterol (LDL-C) in phase 1 trials. We assessed the efficacy and safety of various doses and dosing intervals of REGN727, a monoclonal antibody to PCSK9, added to statins, to further lower LDL-C in patients with heterozygous familial hypercholesterolaemia.
METHODS: This multicentre, randomised, placebo-controlled phase 2 trial was done at 16 lipid clinics in the USA and Canada. Between Jan 18, 2011, and Nov 7, 2011, we enrolled adults with heterozygous familial hypercholesterolaemia and LDL-C concentrations of 2·6 mmol/L or higher on stable diet and statin dose, with or without ezetimibe. Patients were randomly assigned to receive REGN727 150 mg, 200 mg, or 300 mg every 4 weeks, or 150 mg every 2 weeks, or placebo every 2 weeks (ratio 1:1:1:1:1). Randomisation was stratified by concomitant use of ezetimibe at baseline. Investigators, study staff, and patients were masked to treatment group. Blinding was maintained by administration of placebo alternating with REGN727 for the groups of 4 week dosing. The primary endpoint was mean percent reduction in LDL-C from baseline at week 12 and was analysed in the modified intention-to-treat population with an analysis of covariance (ANCOVA) model with treatment group. This trial is registered in ClinicalTrials.gov, number NCT 01266876.
FINDINGS: 77 patients were randomly assigned to study groups (15-16 patients per group) and all were analysed. Least-squares (LS) mean LDL-C reduction from baseline to week 12 was 28,9% (SE 5,08) for 150 mg every 4 weeks (p=0,0113), 31,54% (4,91) for 200 mg every 4 weeks (p=0,0035), 42-53% (5,09) for 300 mg every 4 weeks (p<0,0001), and 67,90% (4,85) for 150 mg every 2 weeks (p<0,0001), compared with 10,65% (5,04) with placebo. One serious adverse event was reported with placebo and none with REGN727. No increases of more than three times the upper limit of normal were reported for hepatic transaminases or creatinine kinase. The most common adverse event was injection-site reaction with one patient in the group of 300 mg REGN727 terminating treatment.
INTERPRETATION: REGN727 was well tolerated and achieved substantial further LDL-C reduction in patients with heterozygous familial hypercholesterolaemia and elevated LDL-C treated with high-dose statins, with or without ezetimibe. REGN727 has the potential to provide optimum control of LDL-C in patients with this disorder.
Area Soci
Eventi
39° Congresso Nazionale
 39° Congresso Nazionale
39° Congresso NazionaleRoma, 23-25 novembre 2025
Save the date




 Spring Meeting Gruppi Giovani SID, SIGG, SIIA, SIMI, SIPREC, SISA
Spring Meeting Gruppi Giovani SID, SIGG, SIIA, SIMI, SIPREC, SISARimini, 6-8 aprile 2025
[continua a leggere]
 SISA LIPID ACADEMY - Corso avanzato di lipidologia clinica
SISA LIPID ACADEMY - Corso avanzato di lipidologia clinicaModena, 4-5 Luglio 2024
[continua a leggere]Giornale Italiano Arteriosclerosi
HoFH today
 Rivista Italiana della
Rivista Italiana della
Ipercolesterolemia
Familiare Omozigote
Anno 6 • N.1/2024
Rivista NMCD
Diateca
[continua a leggere]
[continua a leggere]
Newsletter
il vostro indirizzo di posta elettronica
Progetto LIPIGEN
Nuovo sito dedicato al Progetto LIPIGEN
Progetto LIPIGEN - Vecchio portale
E' necessario essere loggati come utente
Lipigen per poter accedere alla pagina
PROject Statin Intolerance SISA
PROSISA – PROject Statin Intolerance SISA
E' necessario essere loggati come utente
PROSISA per poter accedere alla pagina
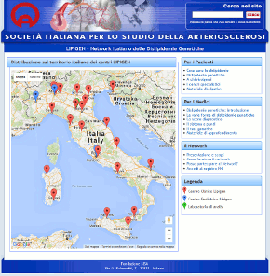
GILA - Lipoprotein Aferesi
Gruppo Interdisciplinare Lipoprotein Aferesi
(Accesso Gruppo GILA-Lipoprotein Aferesi)
E' necessario essere loggati come utente del Gruppo GILA per poter accedere
Gruppo Interdisciplinare Lipoprotein Aferesi
(Documentazione ad accesso libero)
Pagina informativa per medici e pazienti










